文献:
Evaluation of anthocyanins in Aronia melanocarpa/BSA binding by spectroscopic studies
文献链接:
https://amb-express.springeropen.com/articles/10.1186/s13568-018-0604-5作者:
Jie Wei , Dexin Xu, Xiao Zhang, Jing Yang and Qiuyu Wang
原文摘要:
The interaction between Anthocyanins in Aronia melanocarpa (AMA) and bovine serum albumin (BSA) were studied in this paper by multispectral technology, such as fuorescence quenching titration, circular dichroism (CD) spectroscopy and Fourier transform infrared spectroscopy (FTIR). The results of the fuorescence titration revealed that AMA could strongly quench the intrinsic fuorescence of BSA by static quenching. The apparent binding constants KSV and number of binding sites n of AMA with BSA were obtained by fuorescence quenching method. The thermodynamic parameters, enthalpy change (ΔH) and entropy change (ΔS), were calculated to be 18.45 kJ mol−1>0 and 149.72 J mol−1 K−1>0, respectively, which indicated that the interaction of AMA with BSA was driven mainly by hydrophobic forces. The binding process was a spontaneous process of Gibbs free energy change. Based on Förster’s non-radiative energy transfer theory, the distance r between the donor (BSA) and the receptor (AMA) was calculated to be 3.88 nm. Their conformations were analyzed using infrared spectroscopy and CD. The results of multispectral technology showed that the binding of AMA to BSA induced the conformational change of BSA.
牛血清白蛋白(BSA)是血浆蛋白的主要成分之一,由于其与HAS的结构同源性,是研究的血清白蛋白。研究AMA与BSA的结合和相关的能量转移效应。提出这种相互作用的模型,其中BSA的固有荧光被AMA结合进行静态猝灭。
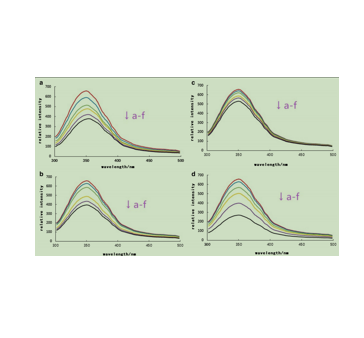
图为:在0、5、10、20、30、40μmol/L(a-f)氰胺-3-O-半乳糖苷(d)的荧光发射光谱
AMA与BSA结合的定性分析可以通过检测荧光光谱来检测。一般来说,蛋白质的荧光体是由蛋白质中存在的三种内在的荧光体引起的,如色氨酸、酪氨酸和苯丙氨酸残基。Te fgure显示激发波长,蛋白质的荧光光谱,BSA的最大荧光发射波长(λmax)。λmax处的Te荧光强度随着花青素浓度的增加而降低,λmax出现红移现象,表明该蛋白中色氨酸和酪氨酸残基附近的微环境增强,疏水性降低。随着阿拉伯苷和葡萄糖苷浓度的增加,BSA的λmax出现蓝移,表明色氨酸残基附近结合腔的极性减弱,二级结构发生变化。
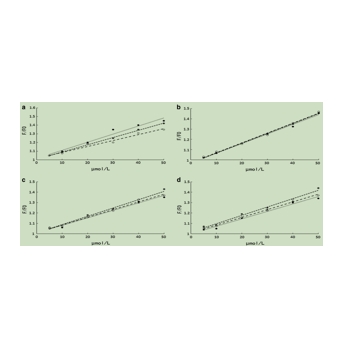
图为:氰胺素-3-O-阿拉伯糖苷(a)、氰胺素-3-O-半乳糖苷(b)、氰胺素-3-O-葡萄糖苷(c)和氰胺苷-3-O-木糖苷(d)在297、317和337 K时的尾形-沃尔默曲线
结果表明,AMA和BSA之间的疏水相互作用在热力学参数的组合中起着主要作用。荧光猝灭表明,AMA可以通过静态机制猝灭BSA的荧光强度,分子对接结果表明,AMA可以与BSA相互作用,而不破坏BSA的二级结构。这两个疏水腔是小分子化合物通过分子建模与蛋白质结合的主要区域,一些花青素具有相同的结合位点。

 2024-12-17 作者:lkr 来源:
2024-12-17 作者:lkr 来源:

