文献:
Enhanced lysosome escape mediated by 1,2-dicarboxylic-cyclohexene anhydride-modified poly-l-lysine dendrimer as a gene delivery system
文献链接:
https://www.sciencedirect.com/science/article/pii/S1818087619308839
作者:
Jianmin Shena, , Jing Chena, Jingbo Maa, Linlan Fanc , Xiaoli Zhang , Ting Yue, Yaping Yana , Yuhang Zhang
相关产品:
Fmoc-NH2-PEG2000-COOH、FA-PEG2000-COOH、DCA
原文摘要:Antisense oligodeoxynucleotide (ASODN) can directly interfere a series of biological events of the target RNA derived from tumor cells through Watson-Crick base pairing, in turn, plays antitumor therapeutic roles. In the study, a novel HIF-1α ASODN-loaded nanocomposite was formulated to efficiently deliver gene to the target RNA. The physicochemical properties of nanocomposite were characterized using TEM, FTIR, DLS and zeta potentials. The mean diameter of resulting GEL-DGL-FA-ASODN-DCA nanocomposite was about 170– 192 nm, and according to the agarose gel retardation assay, the loading amount of ASODN accounted for 166.7 mg/g. The results of cellular uptake showed that the nanocomposite could specifically target to HepG2 and Hela cells. The cytotoxicity assay demonstrated that the toxicity of vectors was greatly reduced by using DCA to reversibly block the cationic DGL. The subcellular distribution images clearly displayed the lysosomal escape ability of the DCA-modified nanocomposite. In vitro exploration of molecular mechanism indicated that the nanocomposite could inhibit mRNA expression and HIF-1α protein translation at different levels. In vivo optical images and quantitative assay testified that the formulation accumulated preferentially in the tumor tissue. In vivo antitumor efficacy research confirmed that this nanocomposite had significant antitumor activity and the tumor inhibitory rate was 77.99%. These results manifested that the GEL-DGL-FA-ASODNDCA nanocomposite was promising in gene therapeutics for antitumor by interacting directly with target RNA.
HIF-1α ASODN片段在体内是非特异性的,容易引起有害作用。而且它可以被组织和细胞中的核酸酶降解,从而导致活性的丧失。此外,裸HIF-1α ASODN片段的细胞内传递也较差,因为带负电荷的ASODN可以诱导对阴离子细胞表面的电荷排斥。特别是,ASODN片段被捕获在溶酶体中并进一步降解。因此,开发一种具有靶向特异性、细胞内化和高效溶酶体逃逸能力等良好特性的基因传递载体。反义寡脱氧核苷酸(ASODN)可以通过沃森-克里克碱基配对直接干扰tumor细胞中靶RNA的一系列生物学事件,进而发挥抗tumor作用。研究用Fmoc-NH2-PEG2000-COOH、FA-PEG2000-COOH、DCA制备了HIF-1αASODN负载纳米复合材料,以有效地将基因传递到目标RNA。
首先制备DGL-NH2和DGL-NH2-FA:在第一次合成中,将Fmoc-NH2-PEG2000-COOH和EDAC溶解在无水二甲亚砜中。将混合物溶液在室温下摇晃加入NHS溶解于无水二甲亚砜中,激活fmoc-nh2-PEG2000-COOH的羧基。将得到的混合物溶液滴加入DGL中,在室温下摇晃,溶解于缓冲液A中。再摇晃后,混合物通过MWCO膜透析,然后冻干获得产品DGL-PEG-NH2-Fmoc(缩写为DGLNH2)。在第二次合成中,将其中一个合成的DGL-NH2再次溶解在缓冲液A中,然后进行超声检查摇晃,随后,通过与EDAC和NHS反应,激活FA-PEG2000-COOH的羧基。然后将活化的FA-PEG2000-COOH滴加入DGL-NH2溶液中,在室温下振荡反应经透析、冻干,该产品用FA-PEG2000-DGL-PEG-NH2-Fmoc(缩写为DGL-NH2-FA)表示。然后制备DGL-NH2-asodn、DGL-NH2-Fa-asodn、DGL-NH2- FA-ASODN-DCA
GEL-DGL-FA-ASODN-DCA、GEL-DGLFA-ASDON和GEL-DGL-ASDON-DCA。采用两步脱溶法合成了明胶纳米颗粒。其次,合成了GEL-DGL-FA-ASODN-DCA、GEL-DGLFA-ASDON和GEL-DGL-ASDON-DCA纳米复合材料。加入EDAC和NHS,激活上述三份GEL悬浮液的羧基。激活的GEL用二甲胺去除保护氨基的Fmoc部分和氨基后,与上述DGL-NH2-FA-ASODN-DCA、DGL-NH2-FA-ASODN和DGL-NH2-ASODN-DCA溶液滴式混合,并在室温下摇匀。通过MWCO为2000 Da的膜透析dH2O,然后冻干,获得最终产品GEL-DGL-FA-ASODN-DCA、GEL-DGL-FA-ASDON和GEL-DGLASODN-DCA。
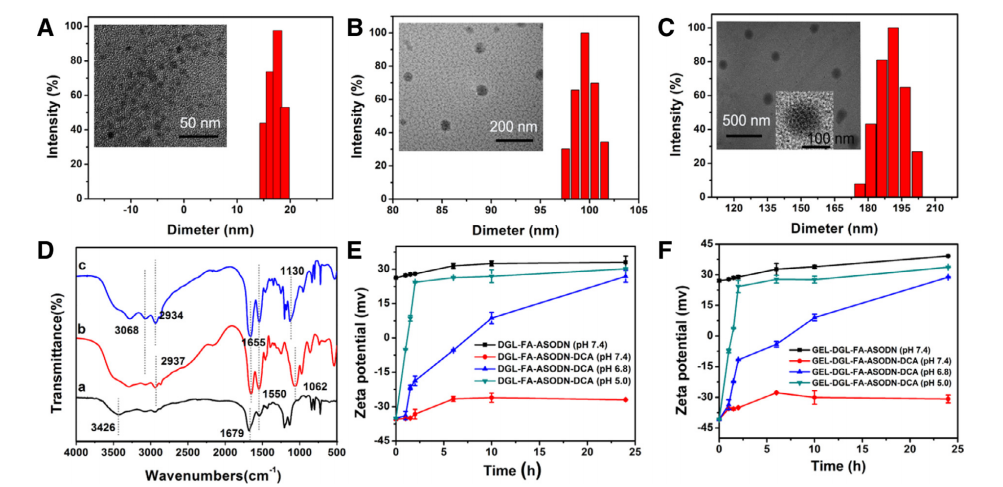
图:DGL-FA-ASODN-DCA的图像及相应的尺寸分布
结论:由Fmoc-NH2-PEG2000-COOH、FA-PEG2000-COOH、DCA制备的纳米复合材料可以特异性地靶向HepG2和Hela细胞。亚细胞分布图像清楚地显示了dca修饰的纳米复合材料的溶酶体逃逸能力。体外分子机制研究表明,该纳米复合材料能在不同水平上抑制mRNA的表达和HIF-1α蛋白的翻译,具有抗tumor活性,抑率为77.99%。这些结果表明,GEL-DGL-FA-DGL纳米复合材料通过与靶RNA直接相互作用,在抗tumor基因Treatment 中具有应用前景。

 2024-12-18 作者:ZJ 来源:
2024-12-18 作者:ZJ 来源:

