文献:Dual-Modified Novel Biomimetic Nanocarriers Improve Targeting and Therapeutic Efficacy in Glioma
文献链接:
作者:Shiyao Fu, Meng Liang, Yuli Wang, Lin Cui, Chunhong Gao , Xiaoyang Chu , Qianqian Liu, Ye Feng , Wei Gong , Meiyan Yang , Zhiping Li , Chunrong Yang , Xiangyang Xie 2, Yang Yang ,
Chunsheng Gao
相关产品:DSPE-PEG2000-Mal 磷脂-聚乙二醇2000-马来酰亚胺
原文摘要:Glioma is a fatal disease with limited treatment options and very short survival.
Although chemotherapy is one of the most important strategies in glioma treatment, it remains extremely clinically challenging largely due to the blood-brain barrier (BBB) and the blood-brain tumor barrier (BBTB). Thus, the development of nanoparticles with both BBB and BBTB penetrability, as well as glioma-targeting feature, is extremely important for the therapy of glioma. New findings in nanomedicine are promoting the development of novel biomaterials. Herein, we designed a red blood cell membrane-coated solid lipid nanoparticle (RBCSLN)-based nanocarrier dual-modified with T7 and NGR peptide (T7/NGR-RBCSLNs) to accomplish these objectives. As a new kind of biomimetic nanovessels, RBCSLNs preserve the complex biological functions of natural cell membranes while possessing physicochemical properties that are needed for efficient drug delivery. T7 is a ligand of transferrin receptors (TfR) with seven peptides that is able to circumvent the BBB and target to glioma. NGR is a peptide ligand of CD13 that is overexpressed during angiogenesis, representing an excellent glioma-homing property. After encapsulating vinca alkaloid vincristine (VCR) as the model drug, T7/NGR-RBCSLNs exhibited the most favourable antiglioma effects in vitro and in vivo by combining the dual-targeting delivery effect. The results demonstrate that dual-modified biomimetic nanoparticles provide a potential
method to improve drug delivery to the brain and hence increasing glioma therapy efficacy.
DSPE-PEG2000-Mal从结构上看,由1,2-二硬脂酰-sn-甘油-3-磷酸乙醇胺(DSPE)、分子量为2000的聚乙二醇(PEG2000)和马来酰亚胺(Mal)组成。DSPE具有很强的亲脂性,能与脂质双分子层相互作用,这使其可用于与细胞膜相关的应用。PEG2000链段具有良好的亲水性和生物相容性,它能提高整个分子在水性环境中的溶解性和稳定性,同时减少非特异性吸附。马来酰亚胺基团(Mal)是一种活性官能团,它可以与含有巯基的生物分子(如蛋白质、多肽中的半胱氨酸残基)发生特异性的化学反应。这种特性使得DSPE-PEG2000-Mal在药物递送系统中可用于连接靶向配体,提高药物对特定细胞或组织的靶向性;在生物成像领域可用于标记生物分子。该文献设计了一种基于红细胞膜包覆的固体脂质纳米颗粒(RBCSLN)的纳米载体,T7和NGR肽(T7/NGR-RBCSLNs),制备过程如下:
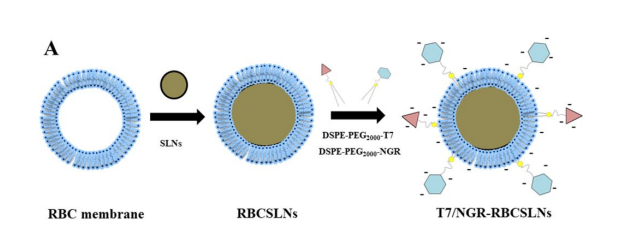
图:T7/NGR-RBCSLNs的合成过程
共轭物的合成与表征
将DSPE-PEG2000-Mal分别偶联到T7和NGR的半胱氨酸残基上,合成DSPE-PEG2000-T7和DSPE-PEG2000-NGR,通过低渗处理收集红细胞膜,采用溶剂注入法制备了sln,接着制备肽修饰rbcsln,采用脂质插入技术对于T7-RBCSLNs的制备,随后将DSPE-PEG2000-肽的伯胺基与荧光探针的羧基偶联,形成目标产物,即DSPE-PEG2000-肽荧光探针。将目标物质(DSPE-PEG2000-NGR-CFDA)冷冻干燥,得到松散的固体粉末。DSPE-PEG2000-T7用5-羧基-x-罗丹明(5-ROX)的荧光探针进行标记,采用与DSPE-PEG2000-NGR-CFDA类似的程序。对含有透析溶液(DSPE-PEG2000-T7-5-ROX)的产品也进行冷冻干燥,得到松散的固体粉末。荧光探针标记的双修饰RBCSLNs(T7-5-ROX/NGR-CFDA-RBCSLNs)的制备方法与T7/NGR-RBCSLNs的制备方法相同,除了DSPE-PEG2000-T7和DSPE-PEG2000-NGR分别被DSPE-PEG2000-T7-5-ROX和DSPE-PEG2000-NGR-CFDA部分取代。
将一定量的DSPE-PEG2000-T7-5-ROX、DSPE-PEG2000-NGR-CFDA或RBCSLNs分别分散在磷酸盐缓冲溶液中,pH值为7.4。采用紫外-可见分光光度计在200~600nm范围内进行光谱扫描,测定了DSPE-PEG2000-T7-CFDA和DSPE-PEG2000-T7-5-ROX的最大吸收波长。制备两种荧光探针的标准溶液,用上述相同的分光光度计测定最大吸收波长(CFDA 493nm,5-ROX578nm)。
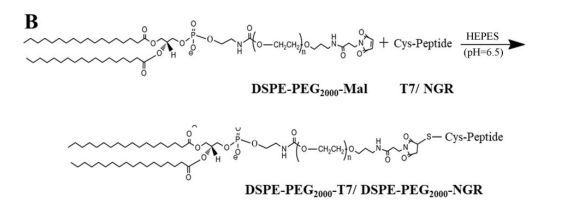
图:DSPE-PEG2000-T7和DSPE-PEG2000-NGR的合成
结论:DSPE-PEG2000-Mal参与制备了双修饰的仿生纳米载体,T7是一种转铁蛋白受体(TfR)的配体,具有7个多肽,能够靶向于Glioma。NGR是CD13的一种肽配体,T7/NGR-RBCSLNs作为模型药物包裹长春花生物碱长春新碱(VCR)后,结合双靶向传递作用,利用T7和NGR肽实现药物靶向。T7/NGR-RBCSLNs可以穿过BBB和BBTB。所制备的经VCR封装的双修饰rbcsln可以有效地提高VCR在细胞的作用。

 2025-04-30 作者:ZJ 来源:
2025-04-30 作者:ZJ 来源:

