文献:Photoinduced PEG deshielding from ROS-sensitive linkage-bridged block copolymer
based nanocarriers for on-demand drug delivery
文献链接:https://www.cqvip.com/doc/journal/2725814875
作者:Liangzhu FengZiliang DongChao LiangMuchao ChenDanlei TaoLiang ChengKai YangZhuang Liu
相关产品:DPPC 二油酰基磷脂酰胆碱
原文摘要:Owing to the existence of severe tumor hypoxia and limited X-ray absorption of solid tumors, the therapeutic efficacy of radiotherapy is far from satisfactory. Herein, ultrasmall iridium nanocrystals (IrNCs) with homogeneous size distribution are successfully synthesized. The obtained IrNCs show catalase-like catalytic activity towards hydrogen peroxide (H2O2) with great temperatures/pH stability. As free IrNCs are prone to be toxified by thiol-containing biomolecules, we encapsulate as-prepared IrNCs within stealth liposomal carriers, obtaining Ir@liposome with well-protected catalytic activity in physiological conditions. By utilizing its efficient photothermal conversion ability, such Ir@liposome shows effective near-infrared-(NIR)-responsive catalytic activity towards H2O2 decomposition. As revealed by in vivo photoacoustic imaging, our Ir@liposome exhibits efficient passive tumor accumulation upon intravenous injection, and could efficiently decompose the tumor endogenous H2O2 into O-2, particularly upon exposure to the NIR laser. As the results of relieved tumor hypoxia after such treatment and the radiosensitization capability of Ir as a high-Z element, greatly enhanced radio-therapeutic efficacy with Ir@liposome is then achieved. This work thus presents a unique type of NIR light controllable theranostic nanozyme based on noble metal nanocrystals as a nanoscale radiosensitizer with great performance in enhancing cancer radiotherapy. (C) 2018 Elsevier Ltd. All rights reserved.
该文献研究显示,在Iridium纳米晶体包封的脂质体中,DPPC(1,2-二酰基-sn-甘油-3-磷酸胆碱)发挥了重要作用,作为近红外光可控的纳米酶,增强了Radiation therapy的效果。DPPC是一种常见的磷脂,应用于生物医学领域,特别是在化合物传递系统中。研究表明,DPPC具有良好的生物相容性和低有害性,能够有效保护包封物质,同时促进其在体内的分布和释放。
在制备Ir@脂质体和Ir-GSH@脂质体时,首先将DPPC、胆固醇和DSPE-mPEG5k的摩尔比为6: 4: 0.5,制备脂质膜,然后用旋转蒸发器干燥。然后,制备的脂质膜用IrNc或Ir-GSH溶液搅拌,然后通过聚碳酸酯过滤器挤压。然后,用PBS作为流动相的G-100柱去除未封装的irnc或Ir-GSH,用MWCO为Amico超滤装置进行压缩。
在此次研究中,DPPC不仅起到了保护Iridium纳米晶体的作用,还大幅度提高了其光热转换效率,使得纳米酶在近红外光照射下表现出催化活性。
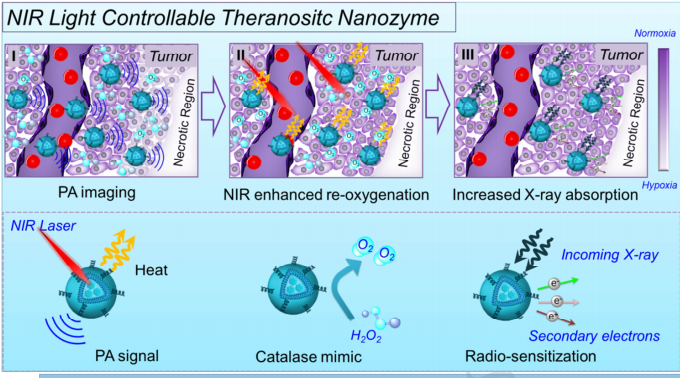
图示1:一种将Ir@脂质体的可控方案。
研究团队通过体外实验和动物模型研究,验证了DPPC包封的Iridium纳米晶体在近红外光照射下能够有效释放能量,从而增强附近tumour组织的放射敏感性。实验结果显示,与传统放射Treatment 相比,结合纳米酶的Treatment 组在tumour抑制率方面表现出了更为的效果。此外,DPPC的应用不仅提升了纳米酶的放Therapeutic effect 果,还降低了对周围健康组织的损伤,进一步提高了Treatment 的安全性。
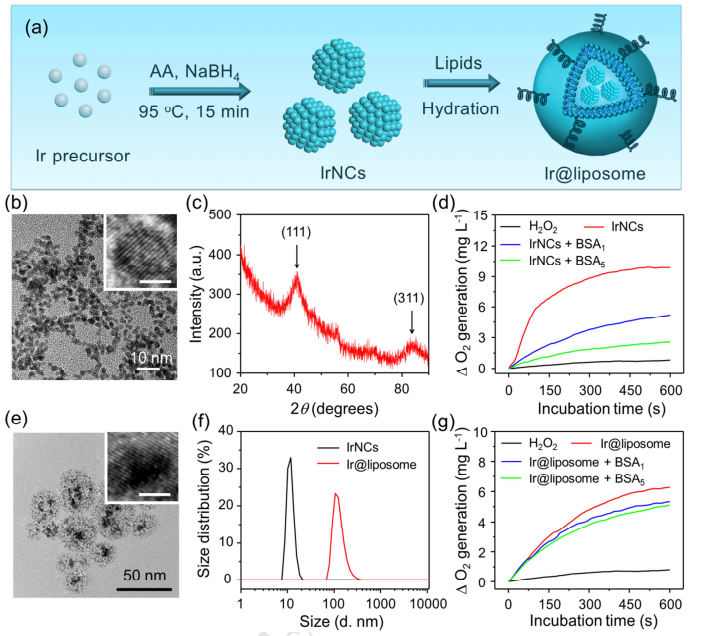
图2:irnc和Ir@脂质体的合成和表征。
结论:DPPC作为主要的脂质成分,增强了脂质体的稳定性和生物相容性,同时改善了包封效果。该研究表明,DPPC能够促进铱纳米晶体的均匀分散,确保其在近红外光照射下的有效催化活性。此外,DPPC的存在有助于实现光驱动的化合物释放,提高了cancer cell对radiation therapy的敏感性。因此,DPPC在该纳米酶系统中不仅增强了tumour靶向性,还带来了效果。

 2025-06-05 作者:wff 来源:
2025-06-05 作者:wff 来源:

