文献:Ultrasound molecular imaging of breast cancer in MCF-7 orthotopic mice using gold nanoshelled poly(lactic-co-glycolic acid) nanocapsules: a novel dual-targeted ultrasound contrast agent
文献链接:https://pubmed.ncbi.nlm.nih.gov/29606871/
作者:Li Xu, Jing Du, Caifeng Wan ,Yu Zhang,Shaowei Xie,Hongli Li , Hong Yang, Fenghua Li
相关产品:SH-PEG-COOH,Mw 2000
原文摘要:
Background: The development of nanoscale molecularly targeted ultrasound contrast agents (UCAs) with high affinity and specificity is critical for ultrasound molecular imaging in the early detection of breast cancer.
Purpose: To prospectively evaluate ultrasound molecular imaging with dual-targeted gold nano shelled poly(lactide-co-glycolic acid) nanocapsules carrying vascular endothelial growth factor receptor type 2 (VEGFR2) and p53 antibodies (DNCs) in MCF-7 orthotopic mice model.
Methods: DNCs were fabricated with an inner PLGA and outer gold nanoshell spherical structure. Its targeting capabilities were evaluated by confocal laser scanning microscopy (CLSM) and flow cytometry (FCM) in vitro. Contrast-enhanced ultrasound imaging (CEUS) with DNCs was evaluated qualitatively and quantitatively in vitro and in MCF-7 orthotopic mice model by two different systems. The biodistribution of NCs in mice was preliminary investigated.Differences were calculated by using analysis of variance.
Results: DNCs showed a well-defined spherical morphology with an average diameter of 276.90±110.50 nm. In vitro, DNCs exhibited high target specificities (79.01±5.63% vs.
2.11±1.07%, P,0.01; 75.54±6.58% vs. 5.21±3.12%, P,0.01) in VEGFR2- and p53-positive
cells compared with control cells. In vivo, CEUS displayed a significantly higher video intensity in two systems using DNCs in comparison with non-targeted PLGA@Au NCs and single-targeted NCs. Biodistribution studies revealed that more DNCs in breast cancer tissue could be detected in mice than in other NCs (P,0.05).Conclusion: DNCs were demonstrated to be novel dual-targeted UCAs and may have potential applications in early non-invasive visualization of breast cancer.
SH-PEG-COOH是由聚乙二醇(PEG)主链和含有巯基(SH)官能化基团的化合物官能化而成,同时还含有羧酸(COOH)官能化基团的共聚物。其化学式可以表示为HS-PEG-COOH。由于SH-PEG-COOH具有良好的生物相容性和稳定性,它被用于制备药物载体和生物材料。该化合物可用于金纳米粒子的修饰,提高溶解性和稳定性,并减少非特异性结合。它还可以用于修饰蛋白质、多肽以及其他材料或者小分子。
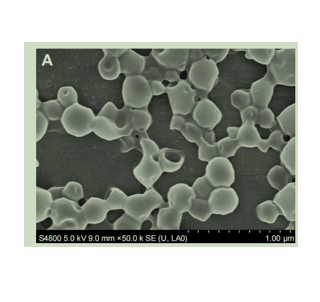
图为:PLGA NCs和DNCs的SEM和TEM
SH-PEG-COOH在PLGA@Aunc中的制备:
将SH-PEG-COOH加入PLGA@Au NCs水溶液中,完全混合,室温搅拌,离心洗涤。将沉淀物重新分散到PBS中,然后分别引入NHS和EDC,激活PLGA@Au-SH-佩库尔NCs表面的羧酸基。在室温下轻轻搅拌,然后重复离心/洗涤步骤,获得活化的PLGA@Au NCs。将溶液重新分散到PBS中,并分成4个等份。用法如下。采用FITC标记的抗p53抗体制备单靶向金纳米壳聚(乳酸共乙醇酸)制备单靶向金纳米壳聚(乳酸共乙醇酸)纳米胶囊。使用等量的适当抗体制备dnc。对照组在溶液中加入L PBS。所有这些制剂都在恒温振荡器中孵育。然后,通过去除游离抗体获得纯靶向的PLGA@Au NCs。
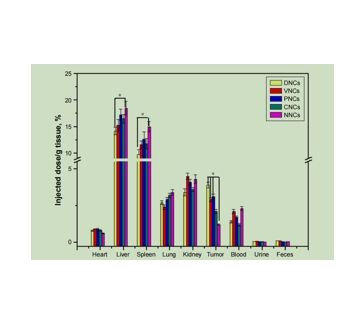
图为:用ICP-AES测定Au的生物分布。
以SH-PEG-COOH作为“桥”,连接抗体和PLGA@Au NCs。在此过程中,采用经典的EDC/NHS激活聚乙二醇化PLGA@Au NCs的羧酸基,通过共价键促进氨基与抗体结合,形成持久的层,作为靶向剂。dnc在Au壳沉积后保持球形,但表面形态粗糙,其平均直径增加。dnc的TEM图像显示,均匀的Au NPs均匀分布在NCs的粗糙表面。DLS的低PI表明dnc的尺寸分布狭窄,均匀性高。

 2024-12-17 作者:lkr 来源:
2024-12-17 作者:lkr 来源:

