文献:A Targeted Chemo-Photodynamic Combination Platform Based on the DOX Prodrug Nanoparticles for Enhanced Cancer Therapy
文献链接:https://pubmed.ncbi.nlm.nih.gov/28378992/
作者:Yumin Zhang, Fan Huang, Chunhua Ren, Lijun Yang, Jianfeng Liu, Zhen Cheng, Liping Chu, and Jinjian Liu
相关产品:
Mal-PEG-CHO 马来酰亚胺-聚乙二醇-醛
原文摘要:Chemo-photodynamic combination therapy has been received widespread
attention in cancer treatment due to its excellent characteristics, such as reducing the adverse side effects of chemo-drugs and improving the therapeutic effects for various cancers. In this study, RGD and DOX was conjugated to PEG by thiol-ene addition and schiff’s base reaction, respectively, to prepare the targeted and pH-sensitive anti-tumor prodrug nanoparticles (RGD-PEG-DOX NPs, RGD-NPs). Subsequently, the photosensitizer chlorin e6 (Ce6) was encapsulated into RGD-NPs, thus obtaining a simple and efficient chemo-photodynamic combination platform (RGD-PEG-DOX/Ce6 NPs, RGD-NPs/Ce6). This nanoparticle possessed high drug loading property of both the chemo-drug and photosensitizer and could simultaneously release them under the mild acidic microenvironment of cancer cells, which was expected to realize the synchronization therapy of chemotherapy and photodynamic therapy (PDT). Compared with free DOX and Ce6, RGD-NPs/Ce6 could significantly improve the cellular uptake capacities of DOX and Ce6, resulting in the increased contents of ROS in cancer cells and effective cytotoxicity for tumor cells (MDA-MB-231 cells and MCF-7 cells) upon a laser radiation. The in vivo experiment showed that RGD-NPs/Ce6 displayed superior tumor targeting, accumulation and retention ability than the other groups (free DOX, free Ce6 and NPs/Ce6), and thus significantly enhancing the anti-tumor effect in vivo with a laser radiation. In addition, the cardiotoxicity induced by DOX was thoroughly wiped out after being loaded and delivered by the nanoparticles according to the pathological analysis. Therefore, the targeted chemo-photodynamic combination therapeutic platform maybe a promising candidate for enhanced cancer therapy.
MAL-PEG-CHO由马来酰亚胺(Maleimide)、聚乙二醇(PEG)和醛基(aldehyde)三部分组成。马来酰亚胺是一个含有碳碳双键和羰基的有机化合物,醛基则是一个含有碳、氧和氢的官能团。聚乙二醇是一种由乙二醇单体聚合而成的聚合物,具有良好的水溶性、生物相容性和稳定性。CRGDK-PEG-DOX是一种特殊的化学结构,通常用于药物递送系统,CRGDK-PEG-DOX能够利用CRGDK环肽的靶向作用,特异性地识别并结合到tumor细胞上,实现药物的准确递送。PEG的引入可以提高药物的稳定性,延长其在体内的循环时间,从而增加药物与tumor细胞的接触机会。通过靶向递送和稳定性增强,CRGDK-PEG-DOX能够减少对正常细胞的损伤,降低有害副作用。
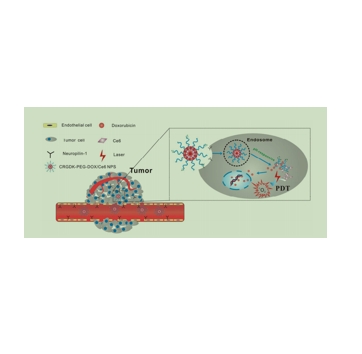
图为:CRGDK-PEG-DOX/Ce6 NPs(RGD-NPs/Ce6)
MAL-PEG-CHO在CRGDK-PEG-DOX共轭物合成中的应用:
MAL-PEG-DOX是由MAL-PEG-CHO和DOX之间的席夫碱基结合而来的。简单地说,将MAL-PEG-CHO和DOX共溶于二甲亚砜(DMSO)中,混合溶液在三乙胺催化下反应。然后,将CRGDK肽直接溶解于DMSO溶液(MAL-PEG-PEG-DOX)中,在室温下放置。将两种反应的溶液用DMSO透析,在温和搅拌的条件下过滤多余的DOX或CRGDK肽,然后用PBS透析,在温和搅拌下消除。经冻干后获得了MAL-PEG-DOX和CRGDK-PEG-DOX,并采用Varian INOVA和UV-vis对其结构进行了表征。采用Maldi-TOF MASS测定MAL-PEG-DOX和CRGDK-PEG-DOX偶联物的分子量。
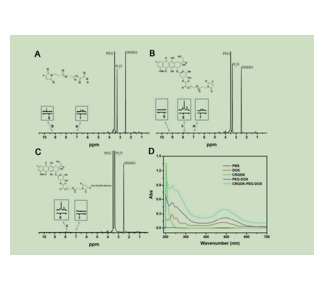
图为:MAL-PEG-CHO的1H NMR谱
结论:采用席夫碱反应和硫醇-烯酰胺化相结合的方法合成了靶向的DOX前药偶联物CRGDK-PEG-DOX。比较图a和图b中的1H NMR谱,以DOX作为对照,发现与DOX的氨基(氨基)反应后,MAL-PEG-CHO中醛基(-CHO)峰消失,产物产生典型峰,证实了schiff碱反应成功合成了MAL-PEG-DOX。然后,从图c中我们可以发现MAL-PEG-DOX与CRGDK肽反应后典型的MAL峰消失,说明CRGDK-PEG-DOX成功偶联。紫外-可见数据进一步证明了CRGDK-PEG-DOX的成功合成。

 2024-12-18 作者:lkr 来源:
2024-12-18 作者:lkr 来源:

