文献:Glioblastoma Therapy Using Codelivery of Cisplatin and Glutathione Peroxidase Targeting siRNA from Iron Oxide Nanoparticles
文献链接:
作者:Yulin Zhang, Xiao Fu, Junsheng Jia, Tobias Wikerholmen, Kaiyan Xi, Yang Kong, Junpeng Wang, Haijun Chen, Yuan Ma, Zhiwei Li, Chuanwei Wang, Qichao Qi, Frits Thorsen, Jian Wang, Jiwei Cui, Xingang Li, Shilei Ni
相关产品:
DSPE PEG2K-Cy7 磷脂-聚乙二醇2k-七甲川花菁染料
原文摘要:Glioblastoma (GBM) is the most common and lethal type of malignant brain tumor in adults. Currently, interventions are lacking, the median overall survival of patients with GBM is less than 15 months, and the postoperative recurrence rate is greater than 60%. We proposed an innovative local chemotherapy involving the construction of gene therapy-based iron oxide nanoparticles (IONPs) as a treatment for patients with glioblastoma after surgery that targeted ferroptosis and apoptosis to address these problems. The porous structure of IONPs with attached carboxyl groups was modified for the codelivery of an siRNA targeting glutathione peroxidase 4 (si-GPX4) and cisplatin (Pt) with high drug loading efficiencies. The synthesized folate (FA)/Pt-siGPX4@IONPs exerted substantial effects on glioblastoma in U87MG and P3#GBM cells, but limited effects on normal human astrocytes (NHAs). During intracellular degradation, IONPs significantly increased iron (Fe2+ and Fe3+) levels, while Pt destroyed nuclear DNA and mitochondrial DNA, leading to apoptosis. Furthermore, IONPs increased H2O2 levels by activating NADPH oxidase (NOX). The Fenton reaction between Fe2+, Fe3+ and intracellular H2O2 generated potent reactive oxygen species (ROS) to initiate ferroptosis, while the coreleased si-GPX4 inhibited GPX4 expression and synergistically improved the therapeutic efficacy through a mechanism related to ferroptosis. As a result, superior therapeutic effects with low systemic toxicity were achieved both in vitro and in vivo, indicating that our nanoformulations might represent safe and efficient ferroptosis and apoptosis inducers for use in combinatorial glioblastoma therapy.
DSPE-PEG2K-FA 磷脂-PEG-叶酸是一种多功能生物分子,DSPE-PEG-FA作为一种化合物载体,可有效地将化合物递送到特定细胞或组织。叶酸受体在许多tumor细胞表面高表达,因此DSPE-PEG-FA可实现对tumor细胞的靶向递送。该文献介绍DSPE-PEG2K-FA制备得到的FA/Pt-si-GPX4@IONPs 纳米颗粒,由顺铂(Pt)、小干扰 RNA(si-GPX4)、氧化铁纳米颗粒(IONPs)和叶酸修饰的脂质体(FA 修饰脂质体)组成,具有良好的生物相容性和抗tumor效果。
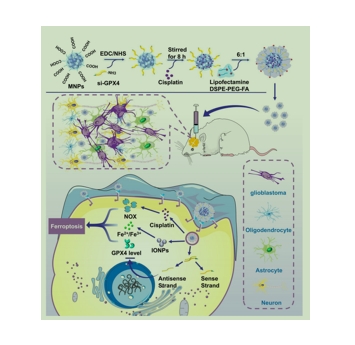
图为:FA/Pt+si-GPX4@IONPs通过诱导铁垂病和细胞Apoptosis 联合Treatment 原位胶质母cytoma 的机制的说明
DSPE-PEG2K-FA在FA/Pt-si-GPX4@IONPs制备和表征中的应用:
通过共沉淀法合成 IONPs。将 si-GPX4 与 IONPs 混合,在一定条件下反应,使 si-GPX4 吸附在 IONPs 表面,得到 si-GPX4@IONPs。将 Pt 与 si-GPX4@IONPs 混合,在一定条件下反应,使 Pt 吸附在 si-GPX4@IONPs 表面,得到 Pt-si-GPX4@IONPs。将 DSPE-PEG2K-FA 与脂质体混合,在一定条件下反应,使 DSPE-PEG2K-FA 插入脂质体膜中,得到 FA 修饰脂质体。将 Pt-si-GPX4@IONPs 与 FA 修饰脂质体混合,在一定条件下反应,使 Pt-si-GPX4@IONPs 被包裹在 FA 修饰脂质体中,得到 FA/Pt-si-GPX4@IONPs1。使用动态光散射仪(DLS)测量 FA/Pt-si-GPX4@IONPs 的粒径和电位。使用透射电子显微镜(TEM)观察 FA/Pt-si-GPX4@IONPs 的形态。将 FA/Pt-si-GPX4@IONPs 在不同条件下储存,观察其粒径和电位的变化。将 FA/Pt-si-GPX4@IONPs 在不同 pH 值和温度下进行化合物释放实验,观察其化合物释放规律。
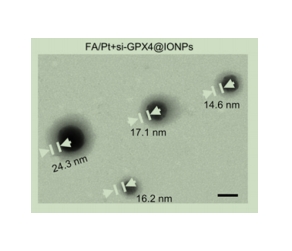
图为:FA/Pt+si-GPX4@IONPs的TEM图像
结论:DSPE-PEG2K-FA 在 FA/Pt-si-GPX4@IONPs 的制备和表征中发挥着重要作用,DSPE-PEG2K-FA 中的叶酸(FA)部分能够特异性地与tumor细胞表面过表达的叶酸受体结合,使制备出的 FA/Pt-si-GPX4@IONPs 纳米颗粒具有主动靶向tumor细胞的能力。通过动态光散射(DLS)等技术可以测量含有 DSPE-PEG2K-FA 的 FA/Pt-si-GPX4@IONPs 的粒径分布和表面电位。

 2025-05-19 作者:lkr 来源:
2025-05-19 作者:lkr 来源:

