文献:Low-Intensity Focused Ultrasound-ResponsiveFerrite-Encapsulated Nanoparticles for Atherosclerotic Plaque Neovascularization Theranostics
文献链接:https://onlinelibrary.wiley.com/doi/10.1002/advs.202100850
作者:Jianting Yao, Zhuowen Yang, Liandi Huang, Chao Yang, Jianxin Wang, Yang Cao, Lan Hao,
Liang Zhang, Jingqi Zhang, Pan Li, Zhigang Wang, Yang Sun,* and Haitao Ran*
相关产品:复合氧化物铁酸锰 MnFe2O4 NPs
原文摘要:
Pathological angiogenesis is a crucial factor that causes atherosclerotic plaque rupture. Sinoporphyrin sodium-mediated sonodynamic therapy (DVDMS-SDT) induces regression of plaque neovascularization in humans without causing obvious side effects. However, a clinical noninvasive theranostic strategy for atherosclerotic plaque neovascularization is urgently needed. A nanoplatform designed for multimodality imaging-guided SDT in plaque angiogenesis theranostics, termed PFP–HMME@PLGA/ MnFe2O4–ramucirumab nanoparticles (PHPMR NPs), is fabricated. It encapsulates manganese ferrite (MnFe2O4), hematoporphyrin monomethyl ether (HMME), and perfluoropentane (PFP) stabilized by polylactic acid-glycolic acid (PLGA) shells and is conjugated to an anti-VEGFR-2 antibody. With excellent magnetic resonance imaging (MRI)/photoacoustic/ ultrasound imaging ability, the distribution of PHPMR NPs in plaque can be
observed in real time. Additionally, they actively accumulate in the mitochondria of rabbit aortic endothelial cells (RAECs), and the PHPMR NP-mediated SDT promotes mitochondrial-caspase apoptosis via the production of reactive oxygen species and inhibits the proliferation, migration, and tubulogenesis of RAECs. On day 3, PHPMR NP-mediated SDT induces apoptosis in neovessel endothelial cells and improves hypoxia in the rabbit advanced plaque. On day 28, PHPMR NP-mediated SDT reduces the density of neovessels, subsequently inhibiting intraplaque hemorrhage and inflammation and eventually stabilizing the plaque. Collectively, PHPMR NP-mediated SDT presents a safe and effective theranostic strategy for inhibiting plaque angiogenesis.
MnFe₂O₄是一种磁性材料,具有软磁性。软磁性材料在外部磁场作用下容易被磁化,且当磁场消失后磁性也容易消失,这使得 MnFe₂O₄在磁存储、磁传感器等领域有潜在的应用价值。具有一定的导电性,虽然其导电性能相较于金属材料较弱,但在半导体领域仍有一定的应用前景,例如可用于制备半导体器件。在较高温度下仍能保持结构的相对稳定性,但在过高温度下也会发生分解等化学反应。锰和铁元素在不同的化学反应条件下可以表现出一定的氧化性和还原性。例如,在与一些还原剂反应时,MnFe₂O₄中的铁离子和锰离子可能会被还原为低价态的离子。MnFe2O4在许多方面有应用,例如在PFP-HMME@PLGA/MnFe2O4-RamNPs纳米平台的制备。
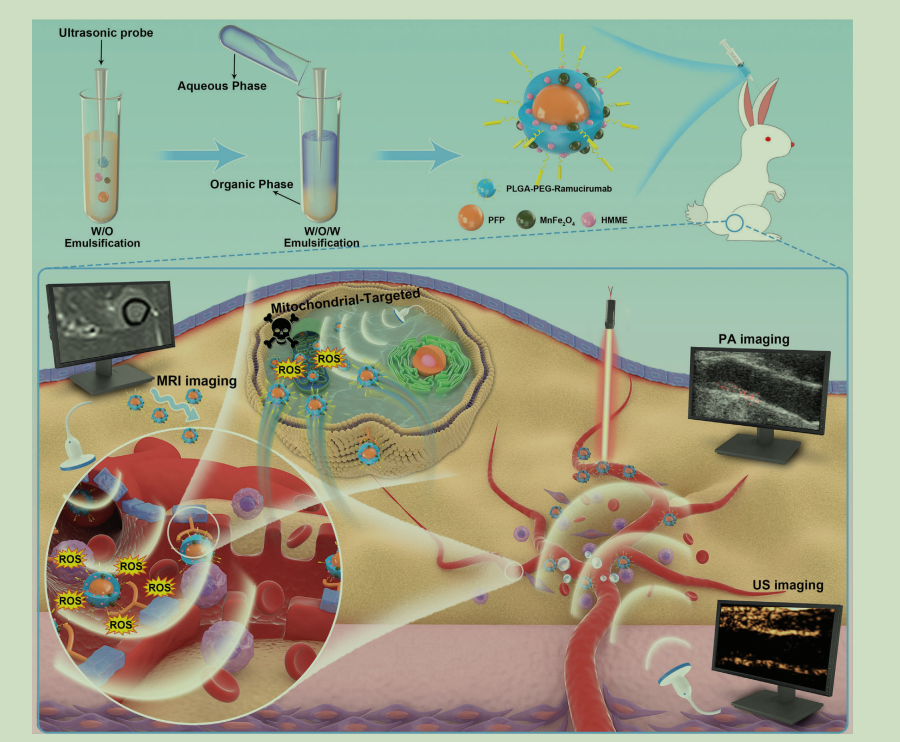
图为:PFP-HMME@PLGA/MnFe2O4-Ram纳米平台的合成过程示意图
PFP-HMME@PLGA/MnFe2O4-RamNPs的制备和表征:
顺磁油酸包覆的 MnFe2O4在紫外-可见范围内表现出吸收。将PFP、HMME和MnFe2O4溶解在含有聚合物PLGA-PEG-拉穆单抗的有机相中,以制备PFP-HMME@PLGA/MnFe2O4-RamNPs。为了优化MnFe2O4的输入量,制备了不同初始体积的MnFe2O4的PFP-HMME@PLGA/MnFe2O4-RamNPs。根据HMME和MnFe2O4的浓度曲线,MnFe2O4的封装效率(EE)和加载能力(LC)提高。同时,HMME的EE和LC上升,随后下降。选择了一个初始MnFe2O4输入量来进行进一步的实验,以平衡NPs的成像能力和声动力学效果。
PFP-HMME@PLGA/MnFe2O4-Ram的体外raec靶向行为:
PFP-HMME@PLGA/MnFe2O4-Ram和HMME、MnFe2O4成功获得,观察发现在一周内的平均直径和zeta电位是相似的,这表明NPs具有稳定性和良好的分散性。PFP-HMME@PLGA/MnFe2O4-RamNPs的透射电镜(TEM)图像显示为球形形态,其中深色的MnFe2O4粒子被纳入PLGA壳层中。用1,3-二苯异苯呋喃(DPBF)和单线态氧传感器绿色(SOSG)O2−和1O2传感器,PFP-HMME@PLGA/MnFe2O4-RamNPs+LIFU分别表现出明显更高的活性氧(活性氧)生产功效,表明其潜在的纳米增敏剂为SDT的。跨NPs的元素线扫描映射显示,Mn、Fe和F存在于这个纳米平台中。PFP-HMME@PLGA/MnFe2O4-RamNPs的吸收波长在UV-vis-NIR区域覆盖范围较广,表明它们可以作为候选的PA造影剂。PFP-HMME@PLGA/MnFe2O4-RamNPs的顺磁特性不仅表明MnFe2O4成功加载,而且NPs作为MRI造影剂的潜力。同时,通过评价PFP-HMME@PLGA/MnFe2O4-RamNPs的LIFU刺激相变特性,微气泡数量增加并达到峰值,随后在减少。这一现象表明,PFP-HMME@PLGA/MnFe2O4-RamNPs可能是转化为LIFU辐照引发的微泡,可作为美国造影剂,微泡下降可能是由于微泡体积在一定程度上膨胀时不稳定所致。
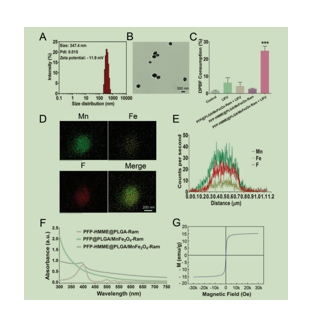
图为:PFP-HMME@PLGA/MnFe2o4-RamNPs的表征
结论:通过将MnFe2O4、HMME和PFP表面修饰封装到PLGA中,制备LIFU响应、线粒体靶向、多功能纳米平台PFP-HMME@PLGA/MnFe2O4-Ram。基于MRI/PA/US多模态成像。纳米声波增敏剂的构建可以在分子水平上实时监测声波增敏剂的分布,使SDT更加准确和高效。

 2025-02-11 作者:lkr 来源:
2025-02-11 作者:lkr 来源:

