文献:Chemoenzymatic Labeling of Extracellular Vesicles for Visualizing Their Cellular Internalization in Real Time
文献链接:https://www.cqvip.com/doc/journal/2724246955
作者:Jiang, YingWang, LeiZhang, PengjuanLiu, XuehuiDi, HuixiaYang, JieLiu, ShuLinPang, DaiWenLiu, Dingbin
相关产品:DOPC 二油酰基磷脂酰胆碱
原文摘要:Extracellular vesicles (EVs) are intercellular communicators that are heavily implicated in diverse pathological processes. However, it is poorly understood how EVs interact with recipient cells due to the lack of appropriate tracking techniques. Here, we report a robust chemoenzymatic labeling technique for visualizing the internalization process of EVs into target cells in real time. This method uses phospholipase D (PLD) to catalyze the in situ exchange of choline by alkyne in the native EV phosphatidylcholine. Subsequent alkyne-azide click chemistry allows conjugation of Cy5 dyes for visualizing EVs internalization by confocal fluorescence microscopy. The fluorescent labeling of EVs was accomplished in an efficient and biocompatible way, without affecting both the morphology and biological activity of EVs. We applied this chemoenzymatic labeling strategy to monitor the cellular uptake of cancer cell-derived EVs in real time and to further reveal multiple internalization mechanisms. This robust, biocompatible labeling strategy provides an essential tool for EV-related studies ranging from chemical biology to drug delivery.
细胞外囊泡是由细胞膜出芽形成的微小膜囊泡,能够携带各种生物分子,如蛋白质、脂质和RNA,在细胞间进行信息传递。传统的标记和追踪方法因灵敏度低和特异性差的问题,限制相关研究。该文献利用DOPC化学酶标记技术,实现对细胞外囊泡的实时可视化,与其在进入目标细胞过程中的动态观察。
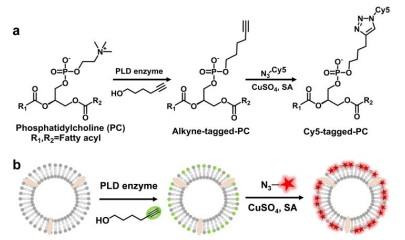
图示1:化学酶促标记策略的说明。
采用了含有二烯丙基磷脂酰胆碱(DOPC)的化学酶标记技术,对提取的细胞外囊泡进行了标记。DOPC作为一种生物相容性强的磷脂,可以有效地嵌入细胞外囊泡的膜中,且不影响其天然属性。研究团队利用酶促反应,将荧光探针引入到囊泡中,形成具有良好荧光特性的标记囊泡。
研究结果表明,DOPC标记的细胞外囊泡在多种细胞类型中的内化效率高于传统标记方法。通过实时成像技术,能够清晰观察到细胞外囊泡的内化过程,包括囊泡与细胞膜的结合、内吞作用及其在细胞内的转运路径。此外,研究还揭示出高内化效率与囊泡类型、尺寸以及细胞接受者的表面特征之间的复杂关系,对细胞间通讯机制的深入理解有重要意义。对化合物递送系统的研究提供新思路。
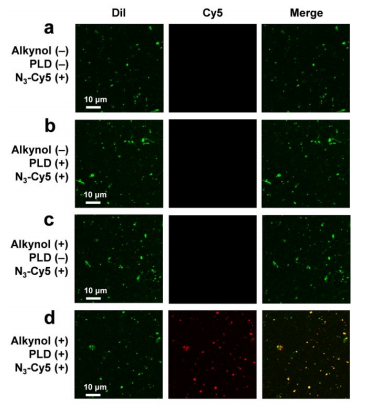
图示2:不同条件下MCF-7细胞分泌的ev经荧光标记后的荧光对比图像。
结论:DOPC化学酶标记技术在外泌体标记和可视化过程中发挥作用。DOPC作为膜成分,帮助构建稳定的外泌体模型,使其标记过程明显。其物理化学性质对外泌体的生物相容性有改善,并促进其在细胞内的摄取。研究结果表明,DOPC修饰的外泌体在荧光标记后的可视化中表现出其稳定性和生物活性,为实时观察细胞对外泌体的内吞过程提供了方法,推动了细胞间信号传递的研究进展。

 2025-05-23 作者:wff 来源:
2025-05-23 作者:wff 来源:

